Abstract
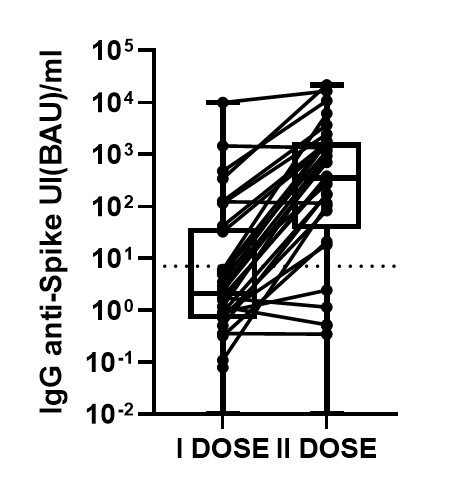
Recipients of allogeneic hematopoietic stem cell transplant (HSCT) have been excluded from clinical trials of SARS-CoV-2 messenger RNA (mRNA) vaccines; however, since these patients are at higher risk of severe complications following infection, they have been given high priority in vaccination campaigns worldwide. In this prospective observational study, we evaluated the immunogenicity of two BNT162b2 (Pfizer-BioNTech) vaccine doses in allogeneic HSCT recipients compared to healthy controls. IgG antibodies to the receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 were analyzed by SARS-CoV-2 IgG II Quant (Abbott, Ireland). The cutoff value of the test used in this study is 7.1 BAU/mL (Binding Antibody Unit/mL) and the results greater than 7.1 indicate that seroconversion has occurred, as recommended by the manufacturer. Peripheral blood samples were collected for immunological analysis at three timepoints: pre-vaccine baseline (w0, before the first BNT162b2 dose), week 3 (w3, before the second vaccine dose) and week 5 (w5, 2 weeks following the second dose). Patients older than 18 years who received BNT162b2 vaccine following an HSCT at seven Italian centers were included in the study. Enrolled patients received two successive doses (at 3-week interval) at a median of 15 months (range 2-141) after HSCT. Twenty-nine age-matched health care workers who were vaccinated with BNT162b2 were recruited as the control group. Among the 34 patients evaluable for serological response, three patients were excluded from the analysis as the baseline serology demonstrated previous natural SARS-CoV-2 infection. On w3, after the first vaccine dose 7/31 (23%) patients developed anti-S IgG antibodies as compared to 28/29 (97%) controls (p<0.01). HSCT recipients showed lower antibody titers (median 1.8 BAU, range 0-481) as compared to healthy controls (median 118 BAU, range 6-1172, p<0.01). In univariate analysis, transplant-to-vaccination interval (>12 months, p<0.01), baseline CD4+ T cell count (>200/mm3, p=0.01), and CD4+CD45RA+ T naive cell count (>100/ mm3, p<0.01) were significantly associated with antibody response after the first vaccine dose. On w5, after the second vaccine dose, 24/31 (77%) of the patients showed antibody response, as compared to 99% of healthy controls (p<0.01); in fact, 71% of non-responders to the first dose developed IgG antibodies after vaccine boost (Figure 1). Median antibody titer after second dose was 350 BAU/ml (0-21.731). In univariate analysis, no significant association was found between patient characteristics and immunogenicity after vaccine boost. Adverse events were rare and modest. Nine percent of the patients reported mild local reactions after vaccine administration, including pain at the injection site and less commonly local erythema, local lymphadenopathy, or swelling; 35% of patients reported systemic adverse events, and all were mild. The most frequently reported systemic reactions included weakness (15%), headache (9%), and diarrhea (3%). In conclusion, in recipients of HSCT, a single dose of the BNT162b2 SARS-CoV-2 vaccine yielded poor efficacy, while immunogenicity increased significantly after vaccine boost at day 21 after the first dose. Patients who received vaccines beyond one year after transplant were more likely to mount anti-S IgG antibodies, which could be due to a broader immune reconstitution, as we observed an enhanced response to single BNT162b2 vaccine dose in patients with higher CD4+ T cell and particularly CD4+CD45RA+ naïve T cell counts.
Kordasti: Alexion: Honoraria; Celgene: Research Funding; Beckman Coulter: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Pane: AbbVie; Amgen; Novartis: Other: Travel, accommodation, expenses; AbbVie; Amgen; Novartis, GSK, Incyte: Speakers Bureau; Novartis Pharma SAS;: Research Funding; AbbVie; Amgen; Novartis, GSK , Incyte: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal